FAQ
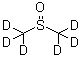
What is the chemical compound dimethyl sulfoxide-D6?
Dimethyl sulfoxide-D6, also known as
DMSO-D6, is a deuterated form of dimethyl sulfoxide that is commonly used in nuclear magnetic
resonance (NMR) spectroscopy. Deuterium is a stable isotope of hydrogen, and DMSO-D6 contains six
deutrium atoms instead of regular hydrogen atoms in the molecule.
How is dimethyl
sulfoxide-D6 used in NMR spectroscopy?
DMSO-D6 is used as a solvent in NMR spectroscopy due
to its ability to dissolve a wide range of organic compounds. It is particularly useful in NMR
studies of biomolecules and other complex organic compounds. The deuterated form of dimethyl
sulfoxide helps to minimize interference from solvent peaks in the NMR spectrum, making it easier to
analyze the signals from the compound of interest.
What are the benefits of using dimethyl
sulfoxide-D6 in NMR spectroscopy?
Using DMSO-D6 as a solvent in NMR spectroscopy offers
several advantages. It has a high solubility for a wide range of organic compounds, making it
versatile for different types of samples. DMSO-D6 is also relatively inert and does not react with
most organic compounds, reducing the risk of chemical interactions during the NMR experiment.
Additionally, the deuterated form of dimethyl sulfoxide helps to improve the quality of NMR spectra
by minimizing interference from solvent peaks.
Are there any limitations or considerations
when using dimethyl sulfoxide-D6 in NMR spectroscopy?
While DMSO-D6 is a useful solvent in
NMR spectroscopy, there are some limitations and considerations to keep in mind. For example,
DMSO-D6 has a relatively high boiling point, which can lead to longer evaporation times during
sample preparation. It is also important to ensure that the NMR tube is properly sealed when using
DMSO-D6 as a solvent, as it can easily evaporate and contaminate the NMR instrument if not handled
correctly.
How should dimethyl sulfoxide-D6 be stored and handled in the
laboratory?
DMSO-D6 should be stored in a cool, dry place away from heat sources and direct
sunlight. It is important to seal the container tightly to prevent evaporation and contamination.
When handling DMSO-D6 in the laboratory, it is recommended to wear appropriate personal protective
equipment, such as gloves and safety goggles, to avoid skin contact and inhalation. Additionally,
DMSO-D6 should be used in a well-ventilated area to minimize exposure to vapors.