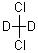FAQ
What is DICHLOROMETHANE-D2 and what is it used for?
DICHLOROMETHANE-D2 is a deuterated form of
dichloromethane, which is a colorless liquid with a sweet odor. It is commonly used as a solvent in
various chemical reactions and processes, such as extraction, purification, and synthesis.
Is
DICHLOROMETHANE-D2 safe to use in laboratory settings?
DICHLOROMETHANE-D2 should be handled with
care in laboratory settings, as it can be harmful if inhaled, swallowed, or in contact with the
skin. Safety precautions should be taken when using DICHLOROMETHANE-D2, such as wearing appropriate
protective equipment and working in a well-ventilated area.
What are some key properties of
DICHLOROMETHANE-D2?
DICHLOROMETHANE-D2 has a boiling point of 40.4°C, a melting point of -95.5°C,
and a density of 1.62 g/cm³. It is soluble in water and most organic solvents, making it a versatile
solvent for various applications in the laboratory.
How is DICHLOROMETHANE-D2 different from
regular DICHLOROMETHANE?
DICHLOROMETHANE-D2 is a deuterated form of dichloromethane, meaning that
it contains deuterium atoms in place of hydrogen atoms. This isotopic substitution can affect
certain chemical properties of the solvent, such as its NMR spectra and reactivity in certain
reactions.
Are there any specific storage requirements for
DICHLOROMETHANE-D2?
DICHLOROMETHANE-D2 should be stored in a cool, dry place away from heat,
sparks, and flame. It should be kept in a tightly sealed container to prevent evaporation and
exposure to moisture. Additionally, DICHLOROMETHANE-D2 should be stored away from incompatible
materials to avoid any chemical reactions.