FAQ
What is DIMETHYL SULFOXIDE-D6?
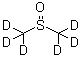
DIMETHYL SULFOXIDE-D6 is a deuterated form of dimethyl
sulfoxide, an organic compound commonly used as a solvent in chemical reactions and pharmaceutical
formulations. The deuterium substitution in DIMETHYL SULFOXIDE-D6 allows for the study of molecular
structures and interactions using NMR spectroscopy.
How is DIMETHYL SULFOXIDE-D6 used in
research?
DIMETHYL SULFOXIDE-D6 is frequently used as a solvent in NMR spectroscopy
experiments due to its high solubility for both organic and inorganic compounds. It is particularly
useful in studies involving small molecules, peptides, and proteins, where the deuterium labeling
helps to simplify NMR spectra and enhance signal resolution.
What are the advantages of using
DIMETHYL SULFOXIDE-D6 in NMR spectroscopy?
One of the main advantages of using DIMETHYL
SULFOXIDE-D6 in NMR spectroscopy is its high solubility for a wide range of compounds. This solvent
is also compatible with most NMR instruments, making it a versatile choice for researchers
conducting molecular studies. Additionally, the deuterium labeling in DIMETHYL SULFOXIDE-D6 helps to
reduce background noise and improve the quality of NMR spectra.
Are there any limitations or
considerations when using DIMETHYL SULFOXIDE-D6 in research?
While DIMETHYL SULFOXIDE-D6 is a
popular choice for NMR spectroscopy, there are some limitations to consider. The deuterium labeling
in this solvent can affect the chemical shifts of certain compounds, so researchers should be aware
of potential interferences in their spectra. Additionally, DIMETHYL SULFOXIDE-D6 is hygroscopic and
can absorb moisture from the atmosphere, which may impact the performance of NMR
experiments.
How should DIMETHYL SULFOXIDE-D6 be stored and handled in the
laboratory?
DIMETHYL SULFOXIDE-D6 should be stored in a cool, dry place away from direct
sunlight and sources of heat. It is also important to keep the container tightly sealed when not in
use to prevent contamination and evaporation. When handling DIMETHYL SULFOXIDE-D6, researchers
should wear appropriate personal protective equipment, such as gloves and safety glasses, to
minimize exposure to the solvent.