FAQ
What is Iodomethane-D3 used for?
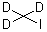
Iodomethane-D3 is a stable, non-radioactive isotope labeled form
of iodomethane which is commonly used in various research applications. Some of the most common uses
of Iodomethane-D3 include labeling experiments to track the movement of molecules in chemical
reactions, as a solvent in nuclear magnetic resonance (NMR) spectroscopy, and as a standard for
calibration purposes in analytical chemistry.
How is Iodomethane-D3 different from regular
iodomethane?
Iodomethane-D3 is a deuterated form of iodomethane, meaning that it contains
deuterium atoms in place of some of the hydrogen atoms in the molecule. This isotopic substitution
can provide important information in NMR experiments, as the presence of deuterium can affect the
spectroscopic properties of the compound. Additionally, Iodomethane-D3 is more stable than regular
iodomethane, making it a preferred choice for certain research applications.
What are the
benefits of using Iodomethane-D3 in NMR spectroscopy?
Iodomethane-D3 is commonly used as a
solvent in NMR spectroscopy due to its chemical properties and stability. When used as a solvent,
Iodomethane-D3 can provide a clean background spectrum, allowing researchers to clearly observe the
signals from their target compound. Additionally, the deuterium atoms in Iodomethane-D3 can help
simplify the NMR spectra by reducing unwanted couplings, making it easier to interpret the
data.
How is Iodomethane-D3 synthesized?
Iodomethane-D3 can be synthesized through a
variety of methods, with one common approach involving the reaction of deuterated iodine with
methane. The deuterated iodine is typically generated by reacting hydrogen iodide with deuterium
gas, producing deuterated iodine which can then be combined with methane under specific conditions
to yield Iodomethane-D3. The synthesis of Iodomethane-D3 requires careful control of reaction
conditions and purification steps to ensure a high-quality product.
Are there any safety
considerations when working with Iodomethane-D3?
As with any chemical compound, it is important
to exercise caution when handling Iodomethane-D3 to ensure the safety of laboratory personnel.
Iodomethane-D3 is a volatile and flammable liquid, so it should be stored and handled in a
well-ventilated area away from potential ignition sources. Additionally, proper personal protective
equipment, such as gloves and safety glasses, should be worn when working with Iodomethane-D3 to
prevent skin contact or inhalation of vapors. It is also important to carefully follow all
recommended storage and disposal procedures to minimize the risk of accidents or environmental
contamination.